Navigate to section
The current industrial landscape is undoubtedly challenging, as in any business, regardless of its nature, aspects such as accuracy, consistency, and efficiency are considered non-negotiable. Achieving these three areas and, at the same time, achieving a goal can be a complex task without the right tools in action.
One technique to handle this complexity is maintaining the operation of documents, commonly called Standard Operating Procedures (SOPs). SOPs include several sections, the most applicable of which are manufacturing work instructions – series of steps that serve as a guide for the execution of complex or recurring tasks, ensuring and improving the safe, agile, and correct execution of processes.
In this guide, let’s discover more about SOP in manufacturing, how to create them, and how to apply them. With our extensive knowledge of manufacturing operations and SOPs, we have included practical tips, examples, and templates here to help you get started with this important tool.
What are Manufacturing SOPs?
The Standard Operation Procedures are documents (traditionally paper-based, although they can also be digital) intended to provide detailed operating instructions and serve as a guide for process execution (usually for operators). These documents provide sequences of actions or step-by-step flow for performing specific tasks or processes, related to the production operation.
Manufacturing SOP documents converts a complex manufacturing process into sequenced instructions with clear steps, practical details, and understandable results, ensuring improved compliance in process consistency, quality, and safety.
These instructions are commonly associated with manufacturing production orders; however, they focus more on ‘how to do or execute the production task’ than on ‘defining what product to make or in what quantity to do so.
Manufacturing SOP also termed as Operational Method Sheets is a hierarchical organization, where:
- A procedure defines the general framework for the execution of a key process and typically includes instructions and records.
- Work instructions, for their part, provide step-by-step execution of the task.
- Finally, their execution entails keeping records, which are like documentary evidence of the correct execution of the instructions.

Nearly 70% of Manufacturing units are shifting towards eSOPs,
Don’t Stay Behind!
How to Create a Manufacturing SOP?
As you might imagine, defining a single way to create effective instructions for the manufacturing process is complex. However, we understand that this involves basic actions such as:
- Collaboration
- Observation
- Iteration
These are common characteristics for creating procedures and instructions according to ISO and worldwide best practices, in general is recognized the follow ones:
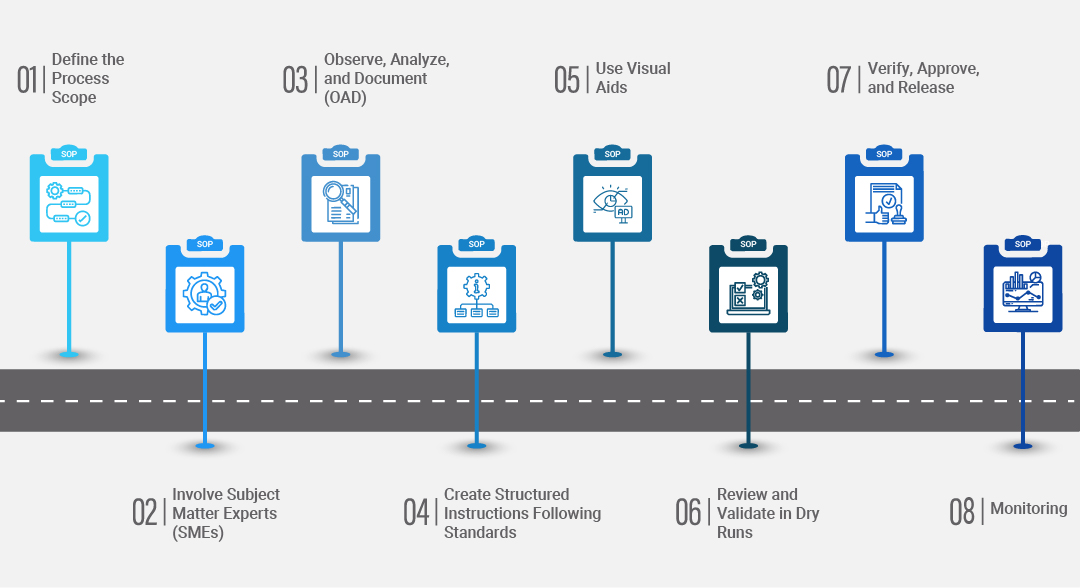
1. Define the Process Scope
The basic step is the identification of the process or task to be documented. This typically covers everything from pre-process steps (such as machine setup) to outputs (such as product assembly or quality inspection). Start by analyzing the overall process.
Tips and tricks: Try to limit where the process itself begins and ends, and what may or may not be considered part of it.
2. Involve Subject Matter Experts (SMEs)
The regular performer of the task typically has the same knowledge, so work closely in a multidisciplinary collaboration involving the personnel involved in the task (such as operators, engineers, and supervisors). Leveraging your team’s knowledge base will ensure you consider hidden conditions and needs, ensuring that instructions reflect real-world needs and practices.
Tips and tricks: Include at least one experienced participant from each profile; this will give your process functional and executional validity.
3. Observe, Analyze, and Document (OAD)
Observe more than one event of the same process, make notes of relevant points, and take evidence such as photos or videos, seeking to capture each step as best as possible. Pay special attention to:
- Sequence of steps
- Type of operation
- Use of tools and materials
- Safety conditions and controls
- Quality controls
- Differences between performers in results (time, number of OK and non-OK products, etc.)
Tips and tricks: Always report your observation activity to supervisors; however, even if you allow the operation to be performed without interruptions or changes, it is common for the execution to be different from what is normally observed.

Access our comprehensive guide to creating and implementing effective Operational Method Sheets, designed to standardize workflows, reduce errors, and enhance quality control.
4. Create Structured Instructions Following Standards
To maintain proper process documentation, keep the content as logical as possible. A typical structure recommended in ISO 9000 includes (but is not limited to):
- Change control (reviewer, approval, release, and version control)
- General title and document ID
- Purpose, scope, and objectives
- Necessary tools and materials
- Applicable operational and safety regulations
- Detailed procedures using a process-instruction-step-by-step structure
- Quality controls
- Problem resolution
Tips and tricks: Use and follow pre-defined international or national standards that will support correct structuring of documents. Check out the template below for reference; it follows ISO 9000 recommendations.
5. Use Visual Aids
A picture says more than a thousand words, so whenever possible, add multimedia files as practical support resources, considering 2D and 3D images, diagrams, and even short audio or video clips to assist your operators in execution. Their use achieves, among other things:
- Reduction in errors
- Significant improvement in comprehension
- Reduction in execution times
- Reduction of language barriers
- Reduction of the learning curve
Tips and tricks: Select the type of element depending on the need to be addressed; for simple tasks, an image is more useful, and for complex tasks, interactive or more visual elements such as videos or 3D images may be more useful.
6. Review and Validate in Dry Runs
Before releasing a procedure, conduct pilot runs and verifications to gather final feedback and adjust (if necessary) the procedure to ensure its clarity and usability.
Tips and tricks: Look for examples of environments that, even if controlled, have most of the conditions that might occur.
7. Verify, Approve, and Release
After completing the documentation, you should conduct a multidisciplinary review (highly recommended) to obtain approval for the document. Define and execute a proper approval process. Only once the document has passed this approval can it be shared and released for productive use. Tips and tricks: Once approved, place it in a centralized location, ideally using manufacturing work instruction software (such as Smart Factory SOPs) to facilitate open access and allow for use while maintaining version control of the document.8. Training and Monitoring
Display the instructions during training sessions, if possible. Also, monitor their use and conduct a comparative analysis of their effectiveness in the workshop versus the previous outcome.
Tips and tricks: Keep a clear monitoring results before and after release of procedures or updates to them is important because it allows you to analyze results and make improvements or understand future or current needs.
While manufacturing units deal with tons of challenges, Smart Factory MOM simplifies the production operations, from execution to product delivery effectively. Remove the hassle of juggling between paper-based documents with Smart Factory MOM
Curious to know HOW?
What are the Key Benefits of Manufacturing SOPs?
SOPs are highly influential and essential in multiple areas for business process optimization. Among the main objectives of SOPs in manufacturing, we can find:
Standardization: Increases adherence to consistent operations, allowing operators to perform tasks in the same way, reducing errors and inconsistencies.
Compliance: Ensures compliance with regulatory requirements, such as Good Manufacturing Practices (GMP) and local and even international standards and regulations (e.g., GMP, ISO).
Training: Can be useful as a reference for employee onboarding during training or initiation, reducing learning curves.
Efficiency: Overall optimization of operations by eliminating unnecessary assumptions and actions.
Quality Control: Increases control, compliance, and monitoring of process and, consequently, product quality through the application of best practices.
Let’s Have a Look at Manufacturing SOPs Document Examples
Here are a few real-world manufacturing work instruction examples across different industries:
Plastics Manufacturing
Why Choose Digital Standard Operating Procedures Integrated into an MES system VS Traditional paper-based ones?
The current era is considered the beginning and development of digital transformation. Companies are increasingly moving away from paper-based standard operating procedures (SOPs) and migrating to systems that allow the use of digital SOPs, especially when they are integrated into execution platforms such as MES (Manufacturing Execution Systems). The reason for this evolution is due to the advantages that these production and monitoring models present. Below, we show a comparison between digital SOPs for manufacturing and traditional paper-based methods:

| Focus area | Digital SOP / in MES | Physical SOP (Paper) |
|---|---|---|
Accessibility |
Remote, Immediate, anytime access and availability from any authorized device. |
Requires stay, move or locate at the place where the file is, meaning there is a physical presence limitation. |
Updateability |
Fast update and release with Real-time changes with version control included. |
Requires multiple actions such as recover previous file, manual reprinting and redistribution and possitioning with high risk of lost version control. |
Traceability |
Automatic traceability and tracking, from logging of who accessed it, and continuous follow up of the execution including full traceability like when, and which version they used. |
Difficult to track; prone to human error and requieres second action to transfer data from record to traceability system. |
Process Integration |
Simple and predefined links to work orders, quality, maintenance, and more. |
Does not integrate with other systems, alwyas require a second activity of data transfer from physical record to traceability system. |
Multimedia |
Commonly allows inclusion of multimedia aids images, videos, and interactive diagrams. |
Limited to printed text and 2D images. |
Access Control |
Remote access with anytime and anyplace availability that Allows defining permissions by role or user. |
Anyone with physical access can view it, what difficult the access control. |
Notifications and Alerts |
If needed, it can send automatic notifications about pending changes or revisions. |
No automatic way to notify changes, all the changes must be notified in place and depends of the time and personnal available. |
Sustainability |
Reduces the use of paper and physical resources. |
Intensive paper and physical space usage, starti ng in his print and continuous maintenance. |
Audits and Compliance |
Facilitates data usage, analysis, audits since use digital records and full traceability on real time |
Commonly have data delays by the tranference to data plataforms manualy and requires manual searching, and documents may be lost. |
Global Standardization |
Support dynamic distribution and ensure all sites, processes, and shifts are working with the same version of the SOP. |
Risk of outdated versions en the same site and in different locations. |
Key Takeaways:
Manufacturing industry is evolving and to keep up the pace with the advancements, a structured system is essential. Digital SOPs in manufacturing let the operators as well as new trainees on the production floor understand the stepwise approach for specific operations. A clear guidance on each complex operation within the manufacturing unit drops the odds of process failure or delays.
To meet today’s industry-leading approach, which requires more dynamic and scalable tracking, consider using software that allows you to create, manage, and distribute instructions digitally. When choosing such a platform, consider that it should provide at least 80% of the points mentioned above. (Learn more about personnel management and how it complements the implementation of work instructions in our SOP Module.
Digitize your operation with eSOPs and enter Industry 4.0 with Smart Factory MOM.
Are paper instructions difficult to maintain and standard operating procedures becoming more obsolete every day? Discover how, using Smart Factory’s SOP and MES modules, you can create, manage, and refine your work instructions, while enhancing them with multimedia visual aids and distributing them throughout your plant or organization with a single click, harnessing the power of real-time information when used minute by minute. Try Smart Factory MES and SOP’s today!A SOP in manufacturing is a document that describes how to perform a specific task or process. It includes both information about the process and instructions and actions (often related to each other). Its use can improve operations by ensuring consistency, quality, and regulatory compliance in a standardized manner.
A Good Manufacturing Practices (GMP) SOP is a documented procedure that considers mandatory or non-mandatory regulatory standards for safety, quality, and efficacy to ensure that manufacturing processes comply both generally and specifically with the applicable standard. These documents typically include detailed steps, quality controls, and safety protocols.
In general, the key components of GMP include:
- Process consistency/standardization
- Equipment and tool validation
- Hygiene and sanitation
- Quality control
- Personnel training
- Documentation and record keeping (evidence)
The easiest difference to recognize is the level of application and its hierarchy, simply put:
- GMP: This is the general regulatory framework (local, international, or global) that defines the principles for ensuring product quality, safety, and integrity. In analogy, they are like a city map, showing the main roads, traffic rules, and speed limits.
- SOPs: These are specific documents derived from GMPs, detailing step-by-step how to execute tasks and actions in accordance with those standards. In this case, they are a GPS route selection, which will guide you step-by-step along a specific route within the road map defined by GMPs, helping you comply with the established limits.
All procedures can undergo changes and improvements, but in general is recommended that you must consider that a procedure must undergo continuous updates under 3 basic conditions: if the applicable regulations change, if the process is modified, if additional conditions exist (such as new equipment or process), or if a gap or problem is detected.



